Completion
Complete each
statement.
|
|
|
1.
|
The concept that matter was composed of tiny indivisible particles was
originally given by ____________________.
|
|
|
2.
|
Researchers discovered a subatomic particle while working with the cathode ray
tube. The particle is known as a(n) ____________________.
|
|
|
3.
|
A ____________________ is a subatomic particle that has mass nearly equal to
that of a proton, but it carries no electrical charge.
|
|
|
4.
|
Atoms with the same number of protons but different number of neutrons are
called ____________________.
|
|
|
5.
|
Uranium is a naturally occurring element that emits particles and rays
spontaneously through a process called _____________________.
|
|
|
6.
|
An alpha particle is _____________________ charged with two protons and
neutrons.
|
|
|
7.
|
In the equation 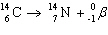 , , the ___________________ decay
of radioactive carbon-14 results in the creation of a new nitrogen-14 atom.
|
|
|
8.
|
A beta particle consists of a fast moving electron and has a(n)
_____________________ charge.
|
|
|
9.
|
In nanotechnology, individual atoms are seen using the ____________________
microscope.
|
|
|
10.
|
The mass of an electron is ____________________ g.
|
|
|
11.
|
James Chadwick showed that the nucleus also contained a neutral subatomic
particle known as the ____________________.
|
|
|
12.
|
Democritus believed that matter is made up of tiny individual particles known as
a(n) ____________________.
|
|
|
13.
|
The number of protons in an atom is called the ____________________ of the
element.
|
Short Answer
|
|
|
14.
|
Write the nuclear equation to describe the alpha decay of 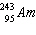 .
|
|
|
15.
|
Compare and contrast mass number and atomic mass. 
|
|
|
16.
|
What is the average atomic mass of this element? | Isotope | Mass (amu) | Percent Abundance | | Phosphorus-29 | 29 | 5% | | Phosphorus-31 | 31 | 71% | | Phosphorus-32 | 32 | 24% | | | |
|
|
|
17.
|
What is the average atomic mass of this element? | Isotope | Mass (amu) | Percent Abundance | | Silver-105 | 105 | 48% | | Silver-108 | 108 | 43% | | Silver-109 | 109 | 9% | | | |
|
|
|
18.
|
Write the chemical symbol for the isotope of cobalt with 37 neutrons.
|
|
|
19.
|
Identify the missing particle in the equation below. Justify your
response. 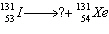
|
|
|
20.
|
Write the nuclear equation to describe the beta-decay of boron-12.
|
|
|
21.
|
Element X has an average atomic mass of 64.32 amu. If this element consists of
only two isotopes, X-64 and X-65, which isotope is present in the greater abundance? Explain how you
can tell.
|
|
|
22.
|
Define an atom.
|
|
|
23.
|
What do you understand by the term atomic mass unit (amu)?
|
|
|
24.
|
What is a nuclear reaction?
|
|
|
25.
|
How are atomic number and mass number denoted in the chemical symbol of the
isotope of an element? Express the shortened notation for an isotope of element X with atomic number
92 and atomic mass 238.
|
|
|
26.
|
Identify the element containing 34 protons.
|
|
|
27.
|
What were the main flaws in Dalton’s atomic theory?
|
|
|
28.
|
Fast moving electrons travel through the empty space surrounding the nucleus.
How are electrons held within the atom?
|